As part of the ongoing pre-normative project PN-VFE, Centexbel is evaluating the possibility of testing surgical masks for their barrier properties against viruses. The aim of this project is to demonstrate the filtration efficiency of different types of masks in relation to very small biological structures, i.e. viruses.
Since early 2020, we have been experiencing a pandemic due to the coronavirus (Covid-19).
This pandemic has severely affected the global economy, the education system, as well as our social relations and well-being.
Knowledge about this virus is still rather patchy in terms of its infectivity, contagion and mode of propagation, even though knowledge about it has greatly evolved over the past year. During this pandemic, it was quickly established that the airborne way was its main mode of transmission, and then the best way to limit its spread was to wear a mouth mask, and more particularly to wear a surgical mask.
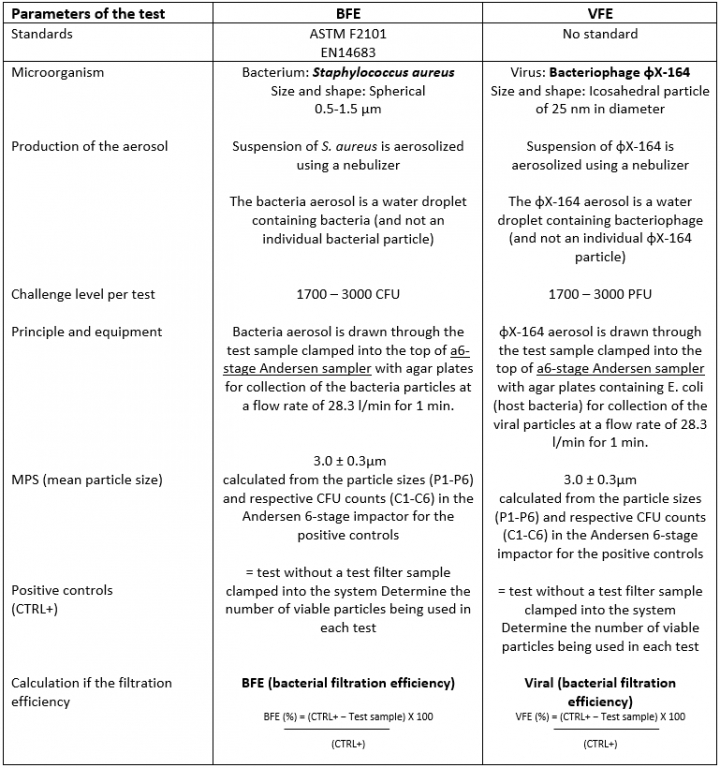
The present European standard EN14683-2019 - surgical masks" sets the requirements in terms of bacterial filtration capacity (BFE), breathability and resistance to blood flow. Worldwide, other standards are based on the same principles.
Surprisingly, there are currently no standards to address the filtration capacity of surgical masks in relation to viruses.
In view of this situation, the study mainly focuses on the problem of masks filtration related to viruses.
To this end, it is proposed to use the BFE technique described in EN14683-2019 while replacing the bacteria with a harmless virus as a first approach.
The idea is to use a very small bacteriophage to simulate viruses and to reveal their passage through the mask by recovering it on an agar containing its host bacteria. The starting point is the equipment currently used to measure the bacterial filtration efficiency as well as the test method itself.
The set-up with the bacteriophages φX-164 has been done and indicates that is possible to follow the protocol used for the BFE.
Comparison between the BFE & VFE protocols
Both methods are currently used in the microbiological lab
The design of the 6-stage Andersen sampler is based on the human respiratory tract, where all airborne particles larger than 0.65μm are classified aerodynamically. The flow rate of 28.3 l/min is similar to the human breathing flow rate to obtain deposition of particles in different stages of the Andersen sampler.
Illustration of the C1-C6 CFU counts of Staphylococcus aureus obtained with the Petri dishes placed in the Andersen 6-stage
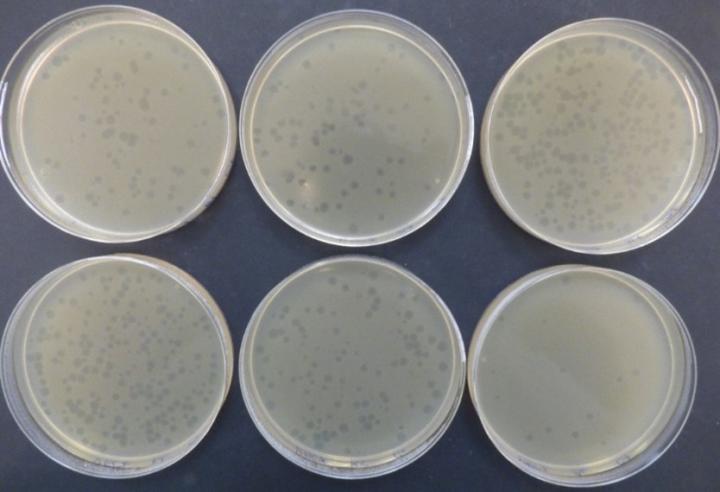
Illustration of the C1-C6 PFU counts of φX-164 (lysis plaques) obtained with the Petri dishes placed in the Andersen 6-stage
Results
At this stage of the research project, we have successfully adapted the BFE method and replaced the Stap. aureus bacteria with a bacteriophage.
The filtration efficiency results of the surgical masks are the same when tested in BFE and VFE following the protocol described above.
This is most likely related to the fact that the aerosol produced is of the same size (MPS) and not of a much smaller size, as is the virus.
The next step in the project is to try to produce an aerosol with a smaller MPS.